The Stark Effect
Half way through my undergrad degree in physics, Prof Ostlie looked up from the Quantum Physics course role and, as if noticing my name for the first time, asked, "Mr. Stark, did you know your name was famous in physics? There is an effect with your name on it." I'd never heard of it before and now it was too late to change majors so I thought I'd better find out what it was. This page is a short description of the spectroscopic phenomena observed by Johannes Stark in 1913, the stark effect. Yes, Dr. Stark's work is the only reason this site's name is any good, otherwise I'd have to make my own physics discovery to put my name on.
What is the Stark Effect
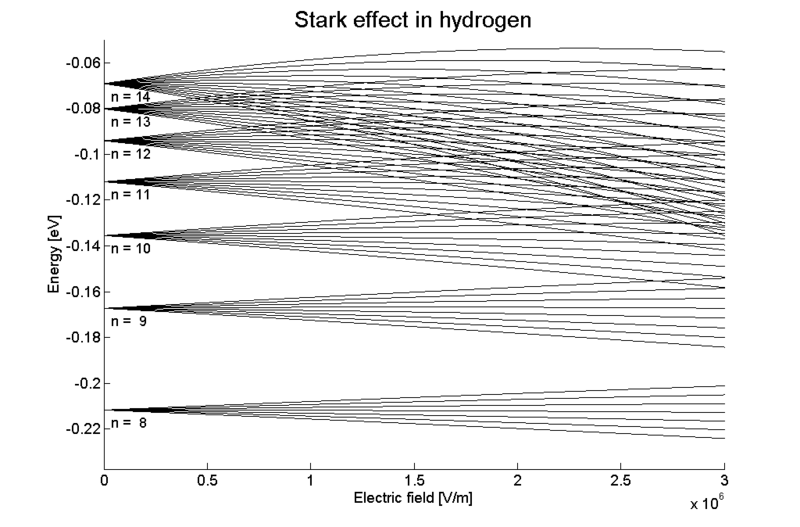 The Stark Effect is shown here in the splitting and shifting of spectral lines in hydrogen under the influence of an external electric field.
The Stark Effect is shown here in the splitting and shifting of spectral lines in hydrogen under the influence of an external electric field.
The stark effect is the shifting and splitting (reduction of degeneracy) of spectral lines in atomic or molecular species under the influence of an externally applied electric field. It is sometimes considered the electric analog to the reduction of degeneracy in atomic and molecular species due to an externally applied magnetic field, the Zeeman effect. I'm not fond of that characterization since the two phenomena are quite different, but it is a reasonable viewpoint.
There are actually two types of stark effect: the linear stark effect and the quadratic version of the stark effect. As expected, the linear stark effect is linearly dependant on the applied electric field while the quadratic stark effect is smaller in the value of splitting and varies as the square of the applied electric field.
History of the Stark Effect
When Zeeman discovered the effect of magnetic fields on the wavelengths of emitted spectra from an excited gas, the Zeeman effect, it sparked a search for a similar effect due to electric fields. For fifteen years the search failed to show such an effect and as a matter of fact, Woldemar Voigt of Göttingen (known for the discovery of the Voigt effect) showed on a theoretical basis, with some assumptions, that no such effect should be expected.
Experimental failure to find the effect of electric fields on emitted spectra was actually due to a very simple phenomenon. Excitation of atoms to show spectra was usually performed by passing an electric arc through a gas. The gas would be ionized allowing current to flow via motion of the freed electrons and collisions between electrons and ions or electrons and neutral atoms would excite the ions or atoms and then spectra would be visible as the ions or atoms decayed back down from their excited states. The problem is that applying an external electric field to this highly conductive gas simply rearranges the charges such that the electric field within the gas is neutralized and you have no appreciable fraction of the emitting ions or atoms experiencing any electric field at all. Johannes Stark, in 1913, recognized the importance of lowering the conductivity of the luminous gas in order to maintain a strong electric field within the gas. His method for accomplishing this was to examine the spectra emitted by the luminous canal rays behind a perforated cathode. Canal rays (anode rays) were discovered by Eugen Goldstein and used by Stark to demonstrate the doppler effect. Stark added a second electrode immediately behind the perforated cathode and applied a strong electric field between this new electrode and that cathode. He found that he could maintain several hundred thousand volts per centimeter between them. He also found that the Hydrogen lines emitted by these canal rays were split into polarized components.
 Johannes Stark won the Nobel Prize for his demonstration of the Stark Effect.
Johannes Stark won the Nobel Prize for his demonstration of the Stark Effect.
When Stark aimed his spectroscope at the region between the electrodes from the side, he found the transverse effect where spectral lines were split into symetrically polarized components on the right and left of the original line. Separation between these components varied linearly with electric field strength, the linear Stark effect. If the field is strong enough, you can also see the quadratic Stark effect in which the separation varies with the square of the applied electric field. With a different arrangement of electrodes, Stark also observed the longitudinal effect parallel to the electric field. Each Balmer line was separated into a number of components. That number increased with the serial number of the line so that the red H line is split into the smallest number (9). Observing perpendicular to the field some of the components seen are polarized parallel to the field while others are polarized perpendicular to the field. Observing in a direction parallel to the electric field, those perpendicularly polarized components appear unpolarized, while the parallel polarized components disappear.
Theory of the Stark Effect
The best way to approach the Stark Effect theoretically is by perturbation theory. The idea is that we have the Hamiltonian of the atom or molecule describing the energies and wavefunctions in the absence of an applied electric field and we simply assume that the change in the Hamiltonian, when we apply an external electric field, can be expressed as a smaller "perturbation" on the main Hamiltonian:
Introduction to the Stark Effect
The Stark effect occurs due to the interaction between the electric field and the electric dipole moment of an atom or molecule. When an external electric field is applied, it perturbs the energy levels of the electrons in the atom, leading to shifts and splits in the spectral lines. These changes can be observed using spectroscopy, providing valuable insights into the structure and behavior of atoms and molecules.
Quantum Mechanical Explanation
In quantum mechanics, the Stark effect can be understood using perturbation theory. The Hamiltonian of an atom in the presence of an electric field E is given by:
where is the unperturbed Hamiltonian of the atom, and is the perturbation due to the electric field. The perturbation term is given by:
Quick Science & Math References
- Our Solar System.
- Earth Facts.
- The Metric System.
- Trigonometric Identities.
- Vector & Tensor Identities.
- Explicit Forms of Vector Operators
- Light & Electromagnetic Spectrum.
- Common Laser Wavelengths.
- Human Physiology Facts.
- Human History Timeline
- Geologic Timeline
- Cambrian Explosion
- Life on Earth Timeline
- ASCII Codes and HTML Display Codes
- Thousands of HTML Symbol Codes
- HTML Symbol Codes for Greek Letters
- The Best Way to put Equations on your Web Page
Physics Basics Series
- Basics of Classical Mechanics.
- Basics of Quantum Mechanics.
- Basics of Electrodynamics.
- Basics of Optics.
- Basics of Mathematical Tools for Physics.
- Basics of Plasma Physics.
- Basics of Solid State Physics.
Math Basics Series
- Numbers
- Arithmetic
- Algebra
- Geometry
- Analysis
Technology Basics Series
- Basics of Remote Sensing.
- Basics of Digital Signal Processing.
- Critical Electronic Circuits
- Infrared Imaging Basics
Knowledge Branches
- Information Theory
- How Reading Works in the Brain
- Psychology of Learning
- Logic
WORK IN PROGRESS
- What is the Stark Effect?
- The Chemistry of Love &/or Addiction
- Critical Thinking: How to question what you see, read or hear.
- Aristotle's Prior Analytics - the birth of Logic.
- Optical Solutions, lenses that solve problems
- Fractals
- PTC - Photon Transfer Curve or Mean Variance Analysis
- 3-D Noise
- Laser Primer
- Rail Guns
- Special Relativity
- Radar Technology
- Acousto-optic Cells
- Harmonic Generation for Laser Frequency Doubling (SHG) and Tripling -using non-linear crystals.
- Measurement: Accuracy & Precision.
- Things you should know about computer modeling of physical phenomena!
- Giant Magneto-resistance
- Peltier Cooling
- Pyro-Electric Detectors
- Piezo-Electric Crystals
- Laser Speckle
- FFT and DFT the fast fourier transform and the discrete fourier transform
- Fabry Perot Etalon
- The Hydrogen Atom.
- PCA (Principal Component Analysis)
- Energy per mass in fuels such as Hydrogen, Gasoline, Kerosene, HMX etc...
- Nobel prize winning work on the CCD
- How does a CCD work and what are the normal characteristics of a CCD
- Nobel Prize Winning work on Giant Magneto-resistance
- FROG -frequency resolved optical gating
- Optical Wavefront Sensing
- THz imaging and time-domain spectroscopy
- Camera Calibration
- Laser Designators
- Resampling
